Abstract
Introduction: Therapeutic plasma exchange (TPE) and corticosteroids have been the foundation of standard-of-care (SOC) treatment for immune thrombotic thrombocytopenic purpura (iTTP) over the last thirty years. Recently, early use of rituximab has emerged as an important adjunct to this SOC regimen. In 2019, the FDA approved caplacizumab for use alongside TPE and immunosuppression in adult patients with iTTP. The clinical efficacy of caplacizumab has been shown to drive its cost-ineffectiveness in the United States (US), with a minimum incremental cost-effectiveness ratio (ICER) of $1.5 million USD per quality-adjusted life-year (QALY). In this context, an open-label, single-arm phase 3 trial (MAYARI, NCT 05468320) will evaluate the use of caplacizumab without first-line TPE in iTTP. The clinical efficacy thresholds that could allow caplacizumab to be cost-effective vs. TPE are unknown. We sought to fill this knowledge gap by conducting the first simulation of a clinical trial of caplacizumab with immunosuppression versus SOC with a focus on an efficacy threshold for 1-year relapse-free survival.
Methods: We built a Markov simulation of patients diagnosed with immune TTP to examine efficacy thresholds to approach cost-effectiveness for treatment with caplacizumab vs. SOC from a US health system perspective, over a time horizon of at least one year. Cost-effectiveness was quantified using an ICER and compared to a prior published threshold of $195,300/QALY. For base case analysis, we assumed SOC achieves the same outcomes as seen with placebo arms of the two randomized clinical trials of caplacizumab, that treatment with caplacizumab with immunosuppression without TPE is as effective as when added to TPE, that treatment in the caplacizumab arm is conducted in the outpatient setting, and that point estimates for decreased mortality from pooled, weighted clinical trial data with caplacizumab are significant. For costs in the caplacizumab arm, medication costs included caplacizumab and 1 cycle of rituximab, assuming all other outpatient costs are $0. Costs in the SOC arm include 1 cycle of rituximab, total number of TPE sessions, intensive care and total hospital stays. We employed age- and sex-adjusted utility values derived using Euroqual-5 Dimensions to capture differential quality-of-life weights associated with inpatient ICU (SOC) vs. outpatient (caplacizumab) arms. We then conducted deterministic sensitivity analyses varying all parameters +/-20%, in addition to a predetermined efficacy threshold analysis to determine a 1-year relapse-free threshold below which caplacizumab-based treatment is cost-effective versus TPE-based therapy. We concluded by performing probabilistic sensitivity analysis that captures uncertainty in all parameters simultaneously over 10,000 Monte Carlo simulations.
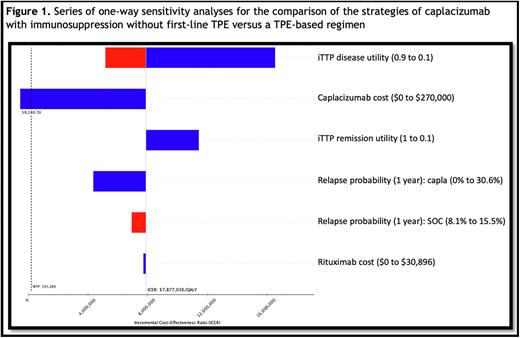
Results: Base-case simulation of caplacizumab- vs. TPE-based therapy in adults with iTTP costs $373,684 versus $105,210, accruing 0.79 and 0.76 QALYs at 1-year of trial follow-up, respectively. A TPE-based regimen generates $43,339 in net monetary benefits, compared to a -$218,478 loss with a caplacizumab-based regimen, which itself is associated with an ICER of $7.8 million USD/QALY. A series of one-way deterministic sensitivity analyses show the only parameter change to modify the ICER by >10% is caplacizumab cost. An efficacy threshold analysis for relapse-free survival shows that the lowest possible cumulative disease relapse probability of 0% by 1 year of follow-up in the caplacizumab arm would be insufficient to render caplacizumab cost-effective (ICER = $4.3 million/QALY), as compared to SOC (Figure 1). The threshold price that allows a caplacizumab-based regimen to approach cost-effectiveness requires a 78% price decrease in caplacizumab cost (to $59,180.70 per course). TPE-based care was the cost-effective strategy in 100.0% of 10,000 simulations.
Conclusion: Proposed treatment with caplacizumab and immunosuppression alone without first-line TPE is cost-ineffective as compared to a standard-of-care with TPE, even if all treatment occurs as an outpatient and a relapse-free 1-year follow up can be achieved with the former. Collecting multiple years of follow-up data, measuring quality-of-life metrics, and ensuring a robust randomized, controlled trial design are paramount before replacing a highly efficacious and proven standard-of-care.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal